Dr. Jerry Silver gently places a large white rat onto a circular table in his lab at Case Western Reserve University.
Bright-eyed and curious, the rat begins exploring. But it doesn't move fast.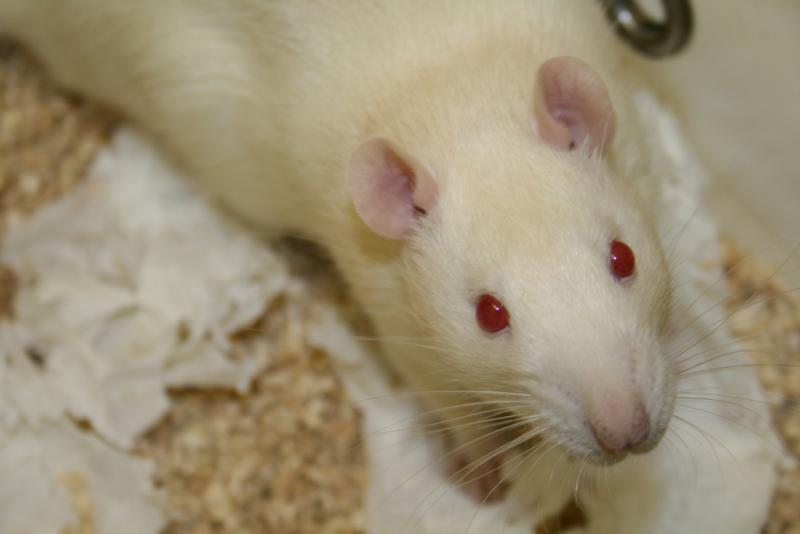
“They get paralyzed just like people," says Silver. He's hoping his experimental technique in healing spinal cord injuries in rats will work in humans.
"If they recover very well that would be wonderful," he says, "and that would be relevant to people.”
The rat eventually wriggles to the table’s edge, dragging its hind legs behind it. It's part of an experiment testing one of Silver’s groundbreaking discoveries on how nerve cells regrow after an injury.
Bridges and enzymes
Silver, with help from a colleague at the Cleveland Clinic, lays a 'bridge' of another nerve type, a peripheral nerve taken from the rat, across the damaged area of the animal's spinal cord.
 He then injects an enzyme called chondroitinase on either end of the bridge to help them interface and "allow the nerve fibers to go in and also come out.” He then injects an enzyme called chondroitinase on either end of the bridge to help them interface and "allow the nerve fibers to go in and also come out.”
Silver has seen some improvement in the rat’s ability to walk and another important process, in urinary function.
Walking, it turns out, is not at the top of the list of abilities that paralyzed people want to regain. It follows other basic needs, says Silver, “especially bladder and bowel function and sexual function."
These, he says, "are very, very important to people who have been paralyzed for long periods of time.”
Progress with an enzyme and a peptide
More than 20 years ago, Silver discovered that chondroitinase helps nerves reconnect. The enzyme is now entering clinical trials at other universities.
But Silver has a new tool in his spinal cord regeneration toolkit. He’s developed a molecule, a peptide, that revs-up the regrowth of nerve cells.
Silver’s hopes are high for improving on the benefits of chondroitinase by itself. He says using just the enzyme alone doesn't significantly improve the rats ability to walk, although they can urinate better. But he believes adding the peptide will "make them walk well and urinate really well!”
The peptide, developed by former grad student Brad Lang, worked surprisingly well in preliminary experiments. So well, says Lang, that Silver "didn’t believe it at all.”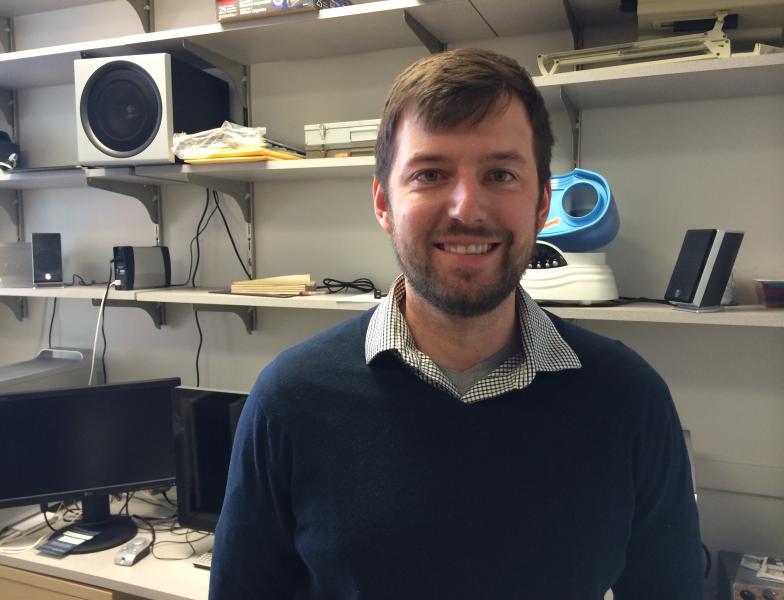
Silver made Lang repeat the experiment five times.
Common sense had told him that injecting the peptide under the skin near the damaged area shouldn’t work. "However this one does,” says Lang.
And when the dose of the peptide was quadrupled, Silver says the results were astounding.
"All animals recover urination after spinal cord injury with the peptide," says Silver. 'Every single animal, 100 percent.”
The team published these results in December. But Silver says there are more surprises ahead.
Unlocking the secrets of Silver's peptide
Silver has put graduate student Amanda Tran in charge of learning how the peptide works. She has cheerfully accepted the challenge to "figure out molecularly what’s going on.”
Silver describes the peptide as the first in a line of dominoes that activates an important downstream chemical signal in the nerve cell.
Now he wants to know "why the last domino falls.”
 And he thinks Tran is close to an answer. "She’s found a downstream event that seems to be triggered quickly but has a long lasting effect," says Silver. And he thinks Tran is close to an answer. "She’s found a downstream event that seems to be triggered quickly but has a long lasting effect," says Silver.
He's not ready to spill the beans on his and Tran's molecular discovery. "It’s a secret,” he says.
But Silver is sure the results will pay off.
“If we could just improve urination in people with spinal-cord injury," he says, "that would be a big advance.”
Progress is painfully slow in the world of spinal cord research. It could be a couple of years before Silver fully understands how his peptide works.
He says he may start working with bigger animals, moving from rats to pigs.
And it will likely be many years before his breakthroughs touch the lives of people living with permanent spinal cord injuries.
But it’s a step in the right direction.
Modulation of CSPG Receptor Promotes Locomotor and Urinary Recovery - Dr. Jerry Silver from Unite 2 Fight Paralysis on Vimeo. |